The ACS Chemistry Exam Formula Sheet is an indispensable tool for students preparing for the challenging American Chemical Society Chemistry Exam. This guide provides a comprehensive overview of the formula sheet, its organization, and effective strategies for using it to maximize exam performance.
Understanding the formula sheet’s structure and locating specific formulas efficiently are crucial. This guide offers practical tips for navigating the sheet and applying formulas to solve exam questions with ease.
Introduction to ACS Chemistry Exam Formula Sheet
The ACS Chemistry Exam Formula Sheet is a valuable resource provided to students during the American Chemical Society (ACS) Chemistry Exam. This formula sheet serves as a concise compilation of essential formulas, constants, and other pertinent information necessary for successfully navigating the exam’s challenging questions.
The primary purpose of the formula sheet is to facilitate efficient problem-solving and save valuable exam time. By having crucial formulas readily available, students can focus their efforts on applying their knowledge and analytical skills rather than wasting time recalling formulas from memory.
The formula sheet ensures a level playing field, as all students have access to the same information, promoting fairness and equity during the exam.
Types of Formulas and Information Included
The ACS Chemistry Exam Formula Sheet encompasses a wide range of formulas and information pertinent to various chemistry subdisciplines, including:
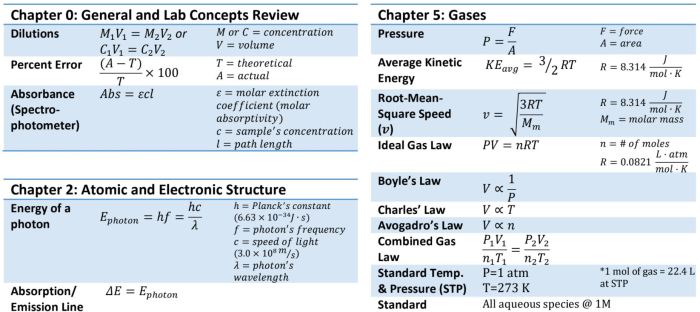
- General chemistry: Fundamental principles, stoichiometry, gas laws, and equilibrium.
- Inorganic chemistry: Nomenclature, periodic trends, and coordination chemistry.
- Organic chemistry: Functional groups, reaction mechanisms, and spectroscopy.
- Analytical chemistry: Equilibrium constants, titration calculations, and instrumental analysis.
- Physical chemistry: Thermodynamics, kinetics, and quantum mechanics.
The formula sheet also includes essential constants such as the Faraday constant, the gas constant, and the Planck constant, among others. Additionally, it provides conversion factors, solubility guidelines, and a periodic table with important atomic data. By incorporating such a comprehensive array of information, the formula sheet empowers students to tackle a diverse range of exam questions with confidence and accuracy.
Understanding the Formula Sheet
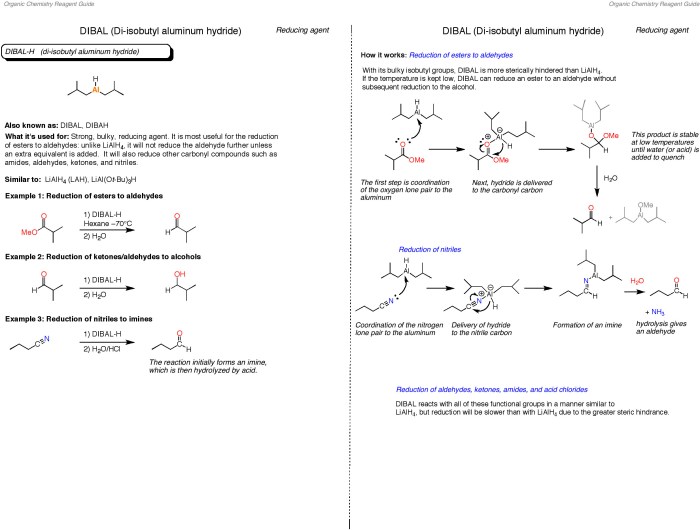
The ACS Chemistry Exam Formula Sheet is meticulously organized to provide efficient access to essential information during the exam. It is structured into sections, each covering specific areas of chemistry, making it easy to locate formulas and data.
Navigating the Formula Sheet
The formula sheet is divided into six sections: Atomic Structure and Properties, Bonding, States of Matter, Equilibrium, Acids and Bases, and Redox Reactions. Each section is further subdivided into s, with formulas and constants clearly labeled. To locate a specific formula, refer to the table of contents or scan the sections for the relevant topic.
Tips for Effective Use
- Familiarize yourself with the formula sheet before the exam to become comfortable with its organization.
- During the exam, utilize the provided time to review the formula sheet and identify any formulas you may need.
- Practice using the formula sheet by solving problems and identifying formulas under timed conditions.
Applying Formulas to Exam Questions: Acs Chemistry Exam Formula Sheet
The ACS Chemistry Exam Formula Sheet is an invaluable tool that can help students succeed on the exam. By understanding how to apply the formulas on the formula sheet to exam questions, students can improve their chances of earning a high score.
There are a number of common exam questions that can be solved using the formula sheet. These questions typically involve calculating the concentration of a solution, the pH of a solution, or the equilibrium constant for a reaction. Students should be familiar with the formulas for these calculations and be able to apply them quickly and accurately.
In addition to knowing the formulas, students should also be familiar with the units that are used in each formula. This will help them to avoid making mistakes when solving exam questions.
Here are some strategies for using the formula sheet to maximize exam performance:
- Become familiar with the formula sheet.The first step to using the formula sheet effectively is to become familiar with its contents. Students should take the time to review the formula sheet and make sure they understand all of the formulas.
- Practice using the formulas.The best way to prepare for the exam is to practice using the formulas on the formula sheet. Students should work through practice problems and make sure they can solve them correctly.
- Use the formula sheet wisely.During the exam, students should use the formula sheet wisely. They should only refer to the formula sheet when they need to and they should make sure they are using the correct formula.
By following these strategies, students can improve their chances of success on the ACS Chemistry Exam.
Common Formulas and Concepts
The ACS Chemistry Exam Formula Sheet provides a comprehensive list of formulas and concepts that are commonly tested on the exam. These formulas cover a wide range of topics, including stoichiometry, thermodynamics, kinetics, and equilibrium. By understanding these formulas and concepts, students can gain a deeper understanding of the fundamental principles of chemistry and improve their performance on the exam.
The formula sheet is organized by topic, making it easy for students to find the information they need. The following table lists some of the most common formulas and concepts covered on the formula sheet, along with examples and explanations:
| Topic | Formula | Example | Explanation |
|---|---|---|---|
| Stoichiometry | $$n = \fracmM$$ | To calculate the number of moles of a substance, divide its mass by its molar mass. | |
| Thermodynamics | $$q = mc\Delta T$$ | To calculate the heat transferred during a temperature change, multiply the mass of the substance by its specific heat capacity and the change in temperature. | |
| Kinetics | $$rate = k[A]^x[B]^y$$ | To determine the rate of a reaction, multiply the rate constant by the concentrations of the reactants raised to their respective orders. | |
| Equilibrium | $$K_c = \frac[C]^c[A]^a[B]^b$$ | To calculate the equilibrium constant for a reaction, divide the concentration of the products raised to their stoichiometric coefficients by the concentration of the reactants raised to their stoichiometric coefficients. |
Advanced Applications of the Formula Sheet
The ACS Chemistry Exam Formula Sheet is not just a compilation of basic formulas; it can be used as a powerful tool for solving complex problems and deriving relationships. By understanding the underlying principles behind the formulas and how they interconnect, students can gain a deeper understanding of chemistry concepts.
One advanced application of the formula sheet is using it to derive relationships between different quantities. For example, the formula for the ideal gas law, PV = nRT, can be rearranged to derive the relationship between pressure and volume at constant temperature (Boyle’s law) or the relationship between temperature and volume at constant pressure (Charles’s law).
Relating Equilibrium Constants, Acs chemistry exam formula sheet
Another advanced application is using the formula sheet to relate equilibrium constants for different reactions. For example, the equilibrium constant for the reaction A + B <=> C can be related to the equilibrium constants for the reactions A <=> B + C and B <=> A + C using the following equation:
Koverall= K 1
K2
where K overallis the equilibrium constant for the overall reaction and K 1and K 2are the equilibrium constants for the individual reactions.
Predicting Reaction Products
The formula sheet can also be used to predict the products of a reaction by considering the relative strengths of acids and bases. For example, the formula for the acid dissociation constant (K a) can be used to predict whether a given acid will donate a proton to a given base.
Tips and Strategies for Using the Formula Sheet

Effective utilization of the formula sheet during the ACS Chemistry Exam is crucial for maximizing your performance. Here are some valuable tips and strategies to help you harness the power of this resource:
Memorization Techniques:
- Spaced Repetition:Review the formula sheet regularly at increasing intervals (e.g., 1 hour, 1 day, 1 week) to strengthen your memory.
- Active Recall:Practice retrieving formulas from memory without looking at the sheet. Use flashcards or quiz yourself to test your recall.
- Chunking:Break down complex formulas into smaller, manageable chunks to facilitate memorization.
Time Management and Efficiency:
- Scan the Formula Sheet Thoroughly:Familiarize yourself with the layout and organization of the sheet before the exam to locate formulas quickly.
- Prioritize Formulas:Identify the most commonly used or important formulas and focus on memorizing them first.
- Use Highlighters and Notes:Highlight key formulas or make notes on the sheet to draw attention to them during the exam.
- Practice Using the Sheet:Solve practice problems under timed conditions to improve your efficiency in finding and applying formulas.
Additional Tips:
- Understand the Formulas:Don’t just memorize formulas; take the time to understand their derivations and applications.
- Practice Unit Conversions:Ensure you are proficient in converting units between different systems, as many formulas require specific units.
- Stay Calm and Focused:During the exam, remain calm and focus on recalling formulas accurately. Avoid panic or rushing.
Commonly Asked Questions
What is the purpose of the ACS Chemistry Exam Formula Sheet?
The formula sheet provides essential formulas and constants needed to solve exam questions, ensuring all students have equal access to this information during the exam.
How should I use the formula sheet effectively during the exam?
Familiarize yourself with the sheet’s organization, locate formulas efficiently, and practice applying them to solve exam-like questions to maximize its effectiveness.
Can the formula sheet help me beyond basic calculations?
Yes, the formula sheet can assist in solving complex problems, deriving relationships, and gaining a deeper understanding of chemistry concepts.