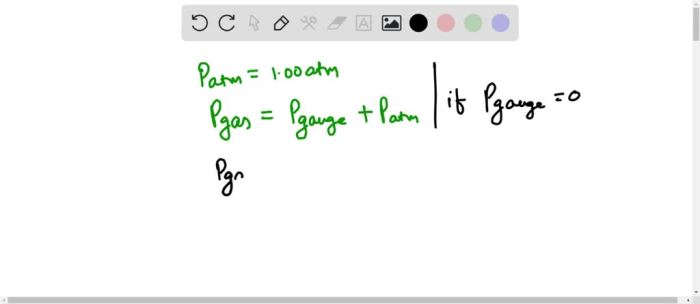
Assume patmos 1.00 atm what is the gas pressure pgas – Assume Patmos 1.00 atm: Determining Gas Pressure (pgas) delves into the fundamental concepts of gas pressure, its measurement, and the factors that influence its behavior. Understanding gas pressure is crucial in various scientific and engineering disciplines, and this article provides a comprehensive exploration of this topic.
The concept of gas pressure is introduced, along with its units of measurement and the relationship between pressure, volume, and temperature. The significance of “Patmos 1.00 atm” is explained, highlighting its role in atmospheric pressure measurement. The ideal gas law is presented as a tool for calculating gas pressure, and its assumptions and limitations are discussed.
1. Define Gas Pressure: Assume Patmos 1.00 Atm What Is The Gas Pressure Pgas
Gas pressure is the force exerted by gas molecules per unit area. It is a measure of the intensity of the molecular bombardment on the surface of an object. The SI unit of gas pressure is the pascal (Pa), which is equal to one newton per square meter (N/m²).
Other common units of gas pressure include the atmosphere (atm), the bar, and the torr.
Gas pressure is affected by three factors: temperature, volume, and the number of gas molecules. According to the ideal gas law, the pressure of a gas is directly proportional to its temperature and the number of gas molecules, and inversely proportional to its volume.
This relationship can be expressed by the following equation:
PV = nRT
where P is the pressure, V is the volume, n is the number of moles of gas, R is the ideal gas constant, and T is the temperature.
2. Patmos 1.00 atm
Patmos 1.00 atm is a standard reference pressure used in many scientific and engineering applications. It is defined as the pressure exerted by a column of mercury 760 mm high at 0°C. This pressure is equivalent to 101,325 Pa or 1.013 bar.
Atmospheric pressure is the pressure exerted by the weight of the air above a given point. It decreases with increasing altitude due to the decreasing density of the air. At sea level, the atmospheric pressure is approximately 1 atm.
3. Calculating Gas Pressure
To calculate the gas pressure, we can use the ideal gas law:
PV = nRT
where P is the pressure, V is the volume, n is the number of moles of gas, R is the ideal gas constant, and T is the temperature.
To use this equation, we need to know the values of the other four variables. If we know the volume, temperature, and number of moles of gas, we can calculate the pressure. If we know the pressure, volume, and temperature, we can calculate the number of moles of gas.
And so on.
4. Factors Affecting Gas Pressure
The pressure of a gas can be affected by several factors, including:
- Temperature:The pressure of a gas is directly proportional to its temperature. As the temperature of a gas increases, the molecules move faster and collide with the walls of the container more often, which increases the pressure.
- Volume:The pressure of a gas is inversely proportional to its volume. As the volume of a gas increases, the molecules have more space to move around, which decreases the pressure.
- Number of gas molecules:The pressure of a gas is directly proportional to the number of gas molecules in the container. As the number of gas molecules increases, the number of collisions with the walls of the container increases, which increases the pressure.
5. Applications of Gas Pressure
Gas pressure has a wide range of applications in various fields, including:
- Engineering:Gas pressure is used in many engineering applications, such as the design of engines, turbines, and pipelines.
- Medicine:Gas pressure is used in medical applications, such as the delivery of anesthesia and the measurement of blood pressure.
- Environmental science:Gas pressure is used in environmental science applications, such as the measurement of air pollution and the study of climate change.
FAQ Compilation
What is the significance of Patmos 1.00 atm?
Patmos 1.00 atm represents standard atmospheric pressure at sea level, which serves as a reference point for pressure measurements.
How does temperature affect gas pressure?
According to the ideal gas law, increasing temperature leads to an increase in gas pressure, assuming volume remains constant.
What are the limitations of the ideal gas law?
The ideal gas law assumes that gas particles are point masses with no intermolecular forces, which may not hold true for all gases under all conditions.