Fe3 po3 2 compound name – Fe3O4, also known as magnetite, is a fascinating compound with a wide range of properties and applications. This article delves into the chemical structure, physical characteristics, chemical reactivity, industrial uses, and natural occurrence of Fe3O4, providing a comprehensive understanding of this remarkable material.
Its chemical formula, Fe3O4, indicates the presence of three iron atoms (Fe) and four oxygen atoms (O) in a specific arrangement. This arrangement results in a unique crystal structure that gives rise to Fe3O4’s distinctive properties.
Chemical Structure

Iron(III) oxide (Fe3O4), also known as magnetite, is a chemical compound composed of iron and oxygen. It is a black, magnetic solid that occurs naturally as the mineral magnetite. The chemical structure of Fe3O4 can be described as a combination of Fe2O3 and FeO, with the iron atoms arranged in an inverse spinel structure.
Arrangement of Iron and Oxygen Atoms
In the inverse spinel structure of Fe3O4, the oxygen atoms form a cubic close-packed (ccp) lattice, while the iron atoms occupy both tetrahedral and octahedral sites within the lattice. The tetrahedral sites are occupied by Fe3+ ions, while the octahedral sites are occupied by both Fe2+ and Fe3+ ions.
The arrangement of iron and oxygen atoms in Fe3O4 can be visualized using a molecular diagram. In this diagram, the oxygen atoms are represented by red spheres, the Fe3+ ions are represented by blue spheres, and the Fe2+ ions are represented by green spheres.
Chemical Formula and Molar Mass
The chemical formula of Fe3O4 indicates that there are three iron atoms for every four oxygen atoms in the compound. The molar mass of Fe3O4 is 231.53 g/mol.
Physical Properties

Fe3O4 exhibits a range of distinct physical properties that contribute to its unique characteristics.
Its appearance is characterized by a deep black color, often described as “magnetic black.” This coloration is attributed to its high iron content and specific crystal structure.
Density
Fe3O4 possesses a relatively high density, typically ranging between 5.18 and 5.24 grams per cubic centimeter (g/cm³). This density is a consequence of its compact crystal structure and the presence of heavy iron atoms.
Melting Point
The melting point of Fe3O4 is exceptionally high, approximately 1597 degrees Celsius (°C) or 2907 degrees Fahrenheit (°F). This remarkable thermal stability is attributed to the strong interatomic bonds within its crystal lattice, making it resistant to melting.
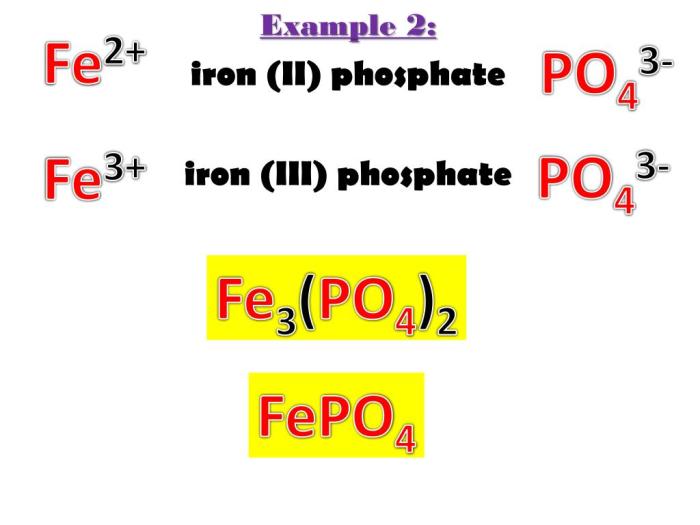
The compound name Fe3PO42- represents an iron(III) phosphate compound. If you’re studying for your APES unit 8 exam, be sure to check out the apes unit 8 study guide for helpful tips and practice questions. Understanding the chemical formula and properties of Fe3PO42- is an important part of the study material.
Magnetic Properties
One of the most notable physical properties of Fe3O4 is its magnetic behavior. It exhibits ferrimagnetism, a unique type of magnetism that arises from the alignment of magnetic moments within its crystal structure. Unlike ferromagnetic materials, which have all magnetic moments aligned in the same direction, ferrimagnetic materials have magnetic moments that are partially aligned in opposite directions, resulting in a net magnetic moment.
| Property | Value |
|---|---|
| Color | Magnetic black |
| Density | 5.18
|
| Melting Point | 1597 °C (2907 °F) |
| Magnetic Behavior | Ferrimagnetic |
Chemical Properties
Fe3O4 is a chemically reactive compound that exhibits both oxidation and reduction properties. It can undergo various chemical reactions under different conditions, such as temperature and pH.
Oxidation and Reduction Reactions
Fe3O4 can be oxidized to Fe2O3 (hematite) under certain conditions, such as high temperatures or the presence of an oxidizing agent. This reaction is typically represented as:
Fe3O4 + 1/2 O2 → 3Fe2O3
Conversely, Fe3O4 can be reduced to FeO (wüstite) under reducing conditions, such as high temperatures or the presence of a reducing agent. This reaction can be represented as:
Fe3O4 + CO → 3FeO + CO2
Stability under Different Conditions
Fe3O4 is generally stable under ambient conditions, but its stability can be affected by temperature and pH. At high temperatures, Fe3O4 can decompose into Fe2O3 and FeO, while at low temperatures, it can be converted to Fe(OH)2 or Fe(OH)3 in the presence of water.
Fe3O4 is also sensitive to pH changes. In acidic solutions, it can dissolve to form Fe3+ ions, while in alkaline solutions, it can precipitate as Fe(OH)3.
Examples of Chemical Reactions
Fe3O4 is involved in various chemical reactions, including:
- Reaction with acids:Fe3O4 + 6HCl → 2FeCl3 + FeCl2 + 3H2O
- Reaction with bases:Fe3O4 + 6NaOH → 2NaFeO2 + 3H2O
- Reaction with reducing agents:Fe3O4 + CO → 3FeO + CO2
- Reaction with oxidizing agents:2Fe3O4 + 1/2 O2 → 3Fe2O3
Industrial Applications

Fe3O4 finds widespread use in various industrial applications due to its unique properties, such as its high magnetic susceptibility, chemical stability, and low cost.
Pigments
Fe3O4 is a valuable pigment in the production of paints, ceramics, and cosmetics. Its natural reddish-brown color and resistance to fading make it an ideal choice for durable and aesthetically pleasing coatings.
Catalysts
Fe3O4 is an effective catalyst in a range of chemical reactions, including the production of hydrogen, ammonia, and methanol. Its magnetic properties allow for easy recovery and reuse, making it a cost-effective and environmentally friendly option.
Magnetic Materials
The high magnetic susceptibility of Fe3O4 makes it suitable for use in magnetic recording devices, such as hard disk drives and magnetic resonance imaging (MRI) systems. It is also used in the production of magnets, magnetic sensors, and magnetic fluids.
Natural Occurrence
Fe3O4, commonly known as magnetite, is a naturally occurring mineral found in the Earth’s crust. It is a black or brownish-black magnetic oxide with the chemical formula FeO·Fe2O3.
Magnetite forms through various geological processes, including:
Igneous Processes
- Magmatic crystallization:Magnetite crystallizes from molten rock (magma) as it cools and solidifies.
- Hydrothermal activity:Magnetite can form as a result of hydrothermal fluids interacting with iron-rich rocks.
Sedimentary Processes
- Chemical precipitation:Magnetite can precipitate from iron-rich solutions in sedimentary environments, such as swamps and lakes.
- Biogenic formation:Certain bacteria can produce magnetite as a byproduct of their metabolism.
Metamorphic Processes, Fe3 po3 2 compound name
- Contact metamorphism:Magnetite can form when iron-rich rocks are subjected to high temperatures and pressures during contact metamorphism.
- Regional metamorphism:Magnetite can also form during regional metamorphism, which involves large-scale deformation and recrystallization of rocks.
Examples of Natural Occurrence
Magnetite is found in various locations around the world, including:
- Kiruna, Sweden (largest known magnetite deposit)
- Magnitogorsk, Russia
- Brazil (Minas Gerais and Bahia regions)
- United States (Iron Range in Minnesota)
- Canada (Labrador Trough)
FAQ Explained: Fe3 Po3 2 Compound Name
What is the chemical formula of Fe3O4?
The chemical formula of Fe3O4 represents three iron atoms (Fe) bonded to four oxygen atoms (O), giving it a ratio of Fe3:O4.
What is the color of Fe3O4?
Fe3O4 is typically black or dark gray in color.
Is Fe3O4 magnetic?
Yes, Fe3O4 is a ferrimagnetic material, meaning it exhibits both ferromagnetic and antiferromagnetic properties, resulting in a net magnetic moment.